
Expanding on the FDA’s FSMA 204 Rule on Food Traceability
In April, I wrote an article about the FDA FSMA 204 Food Traceability Rule. In a critical step toward enhancing food safety, the FDA has issued the final version of FSMA 204, which focuses on strengthening food traceability throughout the supply chain. The goal of FSMA 204 is to enable faster, more effective responses in the event of foodborne illnesses or contamination events, ultimately improving public health outcomes. In this article, I explore the key provisions of the FSMA 204 rule, which foods are affected, and how businesses can use Warehouse Management Systems (WMS) to comply with the new traceability requirements.
Final FSMA 204 Rule: Key Components
The FSMA 204 final rule builds upon previous food safety regulations and establishes critical traceability requirements. Here are the key elements:
Food Traceability List (FTL)
The FSMA 204 rule establishes the Food Traceability List (FTL), which identifies foods subject to additional traceability requirements. The FTL includes high-risk foods that are most susceptible to contamination and pose a greater public health risk. These foods include:
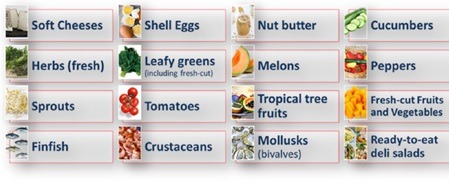
The FDA has determined that these foods, due to their risk profile, require enhanced traceability systems to facilitate quicker identification of sources of contamination if a foodborne illness outbreak occurs.
Key Data Elements (KDEs)
To ensure that traceability records are clear, consistent, and actionable, FSMA 204 requires that key data elements (KDEs) be captured. These KDEs must include specific information for each Critical Tracking Event (CTE), such as:
- Product description (e.g., the type of food or product)
- Lot code (for batch identification)
- Date of receipt and shipment
- Quantity and unit of measure
- Traceability lot code (unique identification for products at each stage of the supply chain)
- Recipient and shipper information
These elements must be recorded at each critical point in the food supply chain—whether it’s production, processing, distribution, or retail.
Critical Tracking Events (CTEs)
CTEs are defined as key points where traceability data must be recorded. These events include activities such as:
- Growing, harvesting, and receiving (for raw agricultural products)
- Processing, manufacturing, and packaging (for foods that undergo treatment)
- Shipping and distribution (for food that moves through the supply chain)
- Retail sale (for food reaching consumers)
For each of these events, businesses must capture and maintain the appropriate KDEs to ensure traceability and compliance.
Requirements for Recordkeeping and Reporting
Under FSMA 204, businesses must maintain traceability records in an electronic format, ensuring data can be accessed, shared, and updated easily. These records must be retained for at least two years. If a foodborne illness outbreak occurs, companies are required to provide traceability data to the FDA within 24 hours.
In cases where a recall is needed, the data should allow the FDA to trace the affected product back through the supply chain as quickly as possible. The use of standardized formats, such as Electronic Data Interchange (EDI), ensures that information can be easily exchanged between partners in the supply chain.
Which Companies Must Comply with FSMA 204 Rule?
The FSMA 204 rule applies to businesses across the food supply chain. This includes:
Food manufacturers and processors: Companies that produce or process foods on the FDA’s Food Traceability List must comply with the traceability requirements.
Distributors and importers: Businesses holding, distributing, or importing the foods listed on the FTL must track and maintain records of these products.
Retailers: Retailers that sell food covered by FSMA 204 must ensure they can trace products from the supplier to the consumer.
The FDA’s regulations aim to ensure that all parties involved in the food supply chain can quickly trace food products and respond to any potential safety concerns.
How a Warehouse Management System (WMS) Can Help
A Warehouse Management System (WMS) can play a vital role in helping companies comply with the FSMA 204 final rule. By automating key processes, a WMS helps businesses track food products across various Critical Tracking Events, capture Key Data Elements, and ensure compliance with the 24-hour reporting requirements. Here’s how a WMS can help:
Real-Time Traceability: A WMS can automatically record critical data at each product lifecycle stage, from receiving and storage to shipping and distribution. This ensures that all relevant Key Data Elements (such as lot codes, dates, and quantities) are captured accurately and consistently.
Integration with Other Systems: WMS platforms can integrate seamlessly with other business systems, including Enterprise Resource Planning (ERP), inventory management systems, and Electronic Data Interchange (EDI) systems. This integration helps ensure that traceability data is shared consistently across the supply chain, making it easier to provide the necessary records to the FDA when required.
Data Accuracy and Efficiency: A WMS can reduce the risk of human error by automating the recording of traceability data. Automated systems are more efficient and accurate, which is crucial for meeting the strict reporting requirements of FSMA 204.
Faster Response Times: When a foodborne illness outbreak occurs, quickly tracing the affected product is critical. A WMS provides a centralized record of all traceability data, allowing businesses to respond to recalls rapidly and effectively. The system can provide instant access to all relevant product details, facilitating rapid identification and removal of contaminated products.
Enhanced Supply Chain Visibility: A WMS offers enhanced visibility into the movement of products throughout the supply chain. This allows businesses to pinpoint potential weaknesses or bottlenecks in the traceability process and address them proactively, reducing the risk of non-compliance and improving overall supply chain efficiency.
Conclusion
The FSMA 204 final rule is a significant step toward strengthening food safety in the United States. Its focus on enhancing traceability and enabling quicker responses to foodborne illnesses will help safeguard public health. Businesses across the food industry, from producers to retailers, must comply with the new requirements by maintaining accurate, accessible traceability records for affected foods.
By implementing a Warehouse Management System (WMS) like Laceup’s WMS, companies can streamline their traceability efforts, automate data capture, and ensure compliance with FSMA 204’s requirements. The right WMS ensures regulatory compliance and provides valuable insights that can enhance food safety, supply chain transparency, and operational efficiency. If you want to learn more, give us your information to schedule a meeting.
I hope this article on FDA FSMA 204 final rule has been helpful to you. I will continue to post information related to warehouse management, distribution practices and trends, and the economy in general. Our channel has a lot of relevant information. Check out this about tracking raw materials in a warehouse.


Sorry, the comment form is closed at this time.