
Essential Features a Vitamin Manufacturers WMS Must Have
Vitamin and nutraceutical producers operate in one of the most demanding supply chains in the world. Unlike traditional food or consumer goods, vitamins face a unique combination of challenges:
- Potency degradation over time
- Regulatory scrutiny (FDA, GMP, FSMA, DSHEA)
- Lot and batch genealogy requirements
- Complex formulations and blending processes
- Short shelf lives and strict quality standards
This is why a generic inventory system is not enough. A Vitamin Manufacturers WMS must be a process-aware, traceability-driven that connects raw material receiving, production, quality control, packaging, and distribution into a single digital workflow. In this article, I explore the must-have features of a WMS tailored for vitamin producers, emphasizing the journey from raw materials to market-ready supplements.
Essential Features of Vitamin Manufacturers WMS
I summarize the main features in this graph.
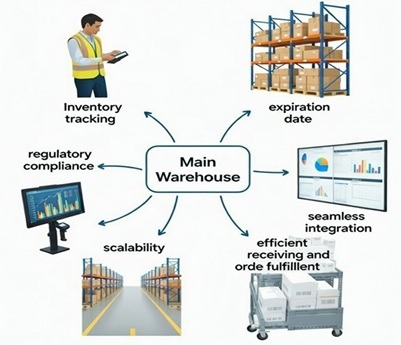
- Advanced Inventory Tracking and Lot Traceability: At the heart of any Vitamin manufacturers WMS is robust inventory management. Raw materials, such as ascorbic acid for vitamin C or botanical extracts, often arrive in bulk and must be tracked meticulously to avoid waste or errors.
- Real-Time Inventory Visibility: The system should provide real-time updates on stock levels, locations, and movements. This includes barcode or RFID scanning for quick identification, reducing human error and enabling cycle counts without halting operations.
- Lot and Batch Tracking: Full traceability is non-negotiable in the supplement industry. The WMS must log every lot from receipt to production, allowing for quick recalls if issues arise, such as contamination or quality deviations. Features like serialized tracking ensure compliance with regulations like the Dietary Supplement Health and Education Act (DSHEA).
For vitamin producers, this feature prevents costly recalls and builds consumer trust by ensuring every bottle can be traced back to its raw material source.
2. Expiration Date and Shelf-Life Management: Raw materials in vitamin production have varying shelf lives; some powders degrade quickly if exposed to moisture, while others require temperature-controlled storage. A WMS must incorporate:
- Automated Expiration Alerts: The system should flag items nearing expiration, prioritizing first-expiry-first-out (FEFO) picking to minimize waste. Integration with sensors for environmental monitoring (e.g., humidity and temperature) ensures proactive alerts.
- Quarantine and Hold Functions: For incoming raw materials awaiting quality checks, the WMS should support quarantine zones. Once approved, materials are released; if rejected, they’re isolated for return or disposal.
This is especially vital for organic or natural vitamin ingredients, where potency can diminish over time, directly impacting product efficacy.
3. Seamless Integration with Quality Control and Production Systems: Vitamin manufacturing isn’t confined to the warehouse; it integrates with production lines. A WMS must integrate with Enterprise Resource Planning (ERP) or Manufacturing Execution Systems (MES) for end-to-end efficiency.
- Quality Assurance Modules: Built-in tools for sampling, testing, and documentation of raw materials upon receipt. The system should automate workflows for Certificate of Analysis (CoA) verification and integrate with lab information management systems (LIMS).
- Production Scheduling Sync: Real-time data exchange ensures raw materials are kitted and delivered to mixing or encapsulation lines just-in-time, reducing downtime and inventory holding costs.
Such integration helps vitamin producers maintain high standards, from blending raw vitamins into tablets to packaging, while adhering to cGMP requirements.
4. Efficient Receiving, Putaway, and Order Fulfillment: Handling raw materials starts at the dock, and a WMS optimized for vitamin producers streamlines these processes:
- Automated Receiving: Support for advance shipping notices (ASNs) to pre-plan receipts, with mobile apps for dock workers to scan and verify shipments against purchase orders.
- Intelligent Putaway: Algorithms that direct storage based on material type, such as storing light-sensitive vitamins in dark zones or hazardous chemicals in segregated areas.
- Picking and Packing Optimization: For finished vitamins, wave picking or zone-based strategies speed up order fulfillment. The system should handle various packaging formats, from bulk drums of raw materials to consumer-ready bottles.
These features reduce labor costs and errors, which is critical in an industry where mislabeling can lead to regulatory fines.
5. Regulatory Compliance and Reporting Tools: The vitamin industry faces stringent oversight, making compliance features indispensable.
- Audit-Ready Documentation: Automated logging of all transactions, with customizable reports for audits. This includes chain-of-custody records for controlled substances or allergens.
- Analytics and KPIs: Dashboards for key performance indicators like inventory turnover, fill rates, and waste percentages. Predictive analytics can forecast raw material needs based on production trends.
By embedding compliance into the WMS, producers can avoid penalties and focus on innovation, such as developing new formulations from sustainable raw materials..
6. Scalability and Customization for Growth: As vitamin producers expand, perhaps into new markets or product lines, the WMS must scale.
- Cloud-Based Flexibility: Modern systems offer cloud deployment for remote access, enabling multi-site management for global supply chains.
- Custom Workflows: Tailorable modules for unique needs, like handling temperature-sensitive shipments or integrating with e-commerce platforms for direct-to-consumer sales.
This ensures the WMS evolves with the business, from small-batch artisanal vitamins to large-scale production.
Conclusion
Vitamin manufacturers WMS is more than software; it is a strategic asset that enhances efficiency, ensures safety, and drives profitability. By prioritizing features like traceability, quality integration, and compliance, manufacturers can navigate the complexities of the supplement industry with confidence. Investing in a robust WMS not only meets current demands but also positions producers for future challenges, such as increased consumer scrutiny of sourcing and sustainability. When selecting a system, evaluate vendors such as Manhattan Associates or SAP for their industry-specific modules, and consider a pilot implementation to test the fit. Ultimately, the right WMS transforms raw materials into reliable, high-quality vitamins that consumers can trust.
At LaceUp Solutions, we explore how technology transforms distribution, from warehouse management and route optimization to digital sales enablement. Subscribe to the LaceUp Blog for weekly insights on wholesale growth, innovation, and the future of logistics. For more information, please get in touch with us to learn about our solutions.
I hope this article on Vitamin manufacturers WMS have been helpful. I will continue to post information related to management, distribution practices and trends, and the economy in general. Our channel has a lot of relevant information. Check out this video on Vitamins Production & Raw Materials Warehouse Tour.


Sorry, the comment form is closed at this time.