
FDA FSMA 204 Food Traceability Rule: Enhancing Supply Chain Transparency
The food industry is undergoing a significant transformation with the introduction of the FDA’s Food Safety Modernization Act (FSMA) 204 food traceability rule. This directive, aimed at enhancing food safety and supply chain transparency, represents a significant step forward in ensuring the safety and security of the food supply chain. In this article, I explore the key aspects of the FDA FSMA 204 rule, which companies must comply with, and how a Warehouse Management System (WMS) can help in achieving compliance.
Key Aspects of the FSMA 204 Directive
The FSMA 204 rule focuses on improving traceability throughout the food supply chain, from farm to table. It requires food companies to maintain electronic records of key data points, including the traceability lot code, product description, and the immediate previous recipient of the product. This information must be readily accessible and searchable to facilitate rapid and accurate tracing of food products in the event of a foodborne illness outbreak or contamination.
Additionally, the rule establishes a standardized format for the exchange of traceability information between trading partners. This format, known as the Electronic Data Interchange EDI 856 transaction set, streamlines the process of sharing traceability data and ensures consistency and accuracy across the supply chain.
Companies Required Complying with FSMA rule 204
The FSMA 204 rule applies to all companies that manufacture, process, pack, or hold food products regulated by the FDA. This includes food producers, processors, distributors, and retailers. Companies that fall under these categories must comply with the requirements of the Food Safety Modernization Act 204, regardless of their size or annual revenue.
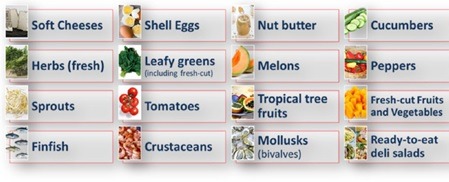
Mandatory category of products
Even though all producers, manufacturers, and distributors must comply with lot traceability, not all products are subject to the strict control associated with Rule 204. The figure shows the list of products with mandatory requirements for this rule. If you are not sure if your products must comply ith the urle, check the FDA Food traceability List (FTL).
Timeline for implementing FSMA section 204 Rule
The FDA initially proposed FSMA rule 204 in September 2020, and it went into effect in January 2023; but businesses have a grace period of two years to ensure compliance with the rule. This means that by early 2025, all businesses that fall under FSMA 204 need to have adjusted their traceability protocols and recordkeeping systems.
FDA rule 204 Requirements
The key requirements of the FDA FSMA 204 Food Traceability Rule include:
Identification of Critical Tracking Events (CTEs): Food manufacturers, processors, packers, and distributors must identify the critical tracking events (CTEs) in their operations, such as growing, receiving, transforming, creating, and shipping.
Record keeping of Key Data Elements (KDEs): Firms are required to establish and maintain records related to key data elements (KDEs), including the traceability product identifier (TPID), the lot code, the quantity produced or packed, and the date of the CTE.
Traceability Lot Codes (TLCs): Each record must contain a lot of code that helps identify the product.
Enhanced Recordkeeping Requirements: The rule requires firms to maintain records related to traceability for at least two years and make them available to the FDA upon request.
How a WMS Can Help Achieve Compliance
A Warehouse Management System (WMS) plays a crucial role in helping companies comply with the FSMA rule.
Record Tracking: A WMS can track and record key data points, such as lot codes and product descriptions, in real-time, ensuring that traceability information is accurate and up-to-date.
Integration: A WMS can integrate with other systems, such as ERP and inventory management systems, to streamline the exchange of traceability information between trading partners. This integration helps ensure that traceability data is consistent across the supply chain and can be easily accessed and shared in the event of a food safety issue.
Items Movement: A WMS can provide valuable insights into the movement of food products throughout the supply chain, allowing companies to identify and address potential traceability issues proactively.
By leveraging the capabilities of a WMS, companies can enhance food safety, improve supply chain transparency, and comply with the requirements of the rule 204 directive.
Conclusion
The FDA Food Safety Modernization Act 204 food traceability rule represents a significant milestone in enhancing food safety and supply chain transparency. Companies in the food industry must ensure compliance with the requirements of the directive to protect consumers and maintain the integrity of the food supply chain. By leveraging the capabilities of a Warehouse Management System (WMS), companies can achieve compliance with the rule 204 and enhance their overall food safety practices. If you want to learn more, give us your information to schedule a meeting.
I hope this article has been helpful to you. I will continue to post information related to warehouse management, distribution practices and trends, and the economy in general. There is a lot of relevant information on our channel. Check this video on How to Track Raw Materials in Your Warehouse.


Sorry, the comment form is closed at this time.