
FSMA 204: How Food Traceability Regulation Will Transform Supply Chains Beyond Food
The FDA’s Food Safety Modernization Act (FSMA) Section 204, or the Food Traceability Rule, is focused on strengthening the U.S. food supply chain. However, its influence is poised to extend far beyond the dinner plate. By mandating a standardized, electronic, and rapid tracking system for high-risk foods, FSMA 204 is establishing a new paradigm for supply chain management that will eventually affect all distributors handling expiration-sensitive products, from pharmaceuticals to cosmetics. In this article, I explore non-food sectors that could be impacted by this rule and how to prepare for that.
Key Aspects of the FSMA 204 Directive
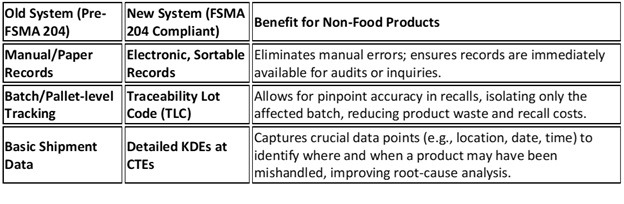
We have written extensively about FSMA 204. This rule focuses on improving traceability throughout the food supply chain, from farm to table. It requires food companies to maintain electronic records of key data points, including the traceability lot code, product description, and the immediate previous recipient of the product. This information must be readily accessible and searchable to facilitate rapid and accurate tracing of food products in the event of a foodborne illness outbreak or contamination. Additionally, the rule establishes a standardized format for exchanging of traceability information between trading partners. This format, known as the Electronic Data Interchange EDI 856 transaction set, streamlines the process of sharing traceability data and ensures consistency and accuracy across the supply chain.
The Broader Impact: From Food to All Perishable Supply Chains
While FSMA 204 targets food, its principles are inspiring traceability enhancements in other sectors where product expiration poses safety or efficacy risks. Industries handling expiration-sensitive products—those that degrade over time, lose potency, or become hazardous—face similar challenges: ensuring integrity, enabling recalls, and maintaining consumer trust.
Pharmaceuticals and Medical Devices:. The Drug Supply Chain Security Act (DSCSA), enacted in 2013, mirrors 204 by requiring package-level traceability for prescription drugs to combat counterfeits and ensure safety. Both regulations emphasize electronic data exchange, lot-level tracking, and rapid response mechanisms. DSCSA mandates serialization (unique identifiers on packages) and traceability from manufacturer to dispenser, including verification of product history, which is crucial for drugs with expiration dates affecting efficacy.
Cosmetics and Consumer Goods: Cosmetics and personal care products, often with limited shelf lives due to microbial growth risks, are another area ripe for influence. While not yet under a direct equivalent to the 204 FSMA rule, global standards like GS1’s Global Traceability Standard (GTS) already apply to non-food consumer goods, including cosmetics. GTS assists in designing traceability systems that track products from raw materials to end-users, ensuring compliance with safety regulations and enabling recalls for expired or contaminated batches. As consumer awareness grows, regulators may push for stricter rules, drawing from FSMA’s model to mandate KDE-like data for ingredients prone to degradation.
Retailer Requirements: Large retailers and major distributors that handle both food and non-food products are leading the charge. Many are already mandating FSMA 204 compliance for all food suppliers, regardless of whether their specific products are on the FTL. It’s a small step for them to extend this practice to other sensitive product categories, making 204-level compliance a de facto industry standard.
The Cost and Benefit of Modern Systems
Distributors who upgrade their systems to meet 204 FSMA rule, will find they have the infrastructure to manage other expiration-sensitive inventory with minimal additional cost.
Implications for Distributors: A Proactive Approach
Distributors, particularly those operating in multiple verticals (e.g., food service, healthcare, and retail), should view FSMA 204 as a foundational shift for the entire supply chain.
Technology Investment and Standardization: Compliance requires moving from fragmented legacy systems to a unified digital solution. Distributors should invest in technology, such as specialized traceability software, blockchain, or advanced ERP systems, that can: Generate and manage TLCs, Capture Data at the “Events”, and Ensure Data Interoperability.
Elevated Personnel Training: A robust traceability system is only as good as the data it receives. Distributors must prioritize training staff on the new Standard Operating Procedures (SOPs) for data capture, particularly for tasks like shipping and receiving, where accurate entry of the TLC and related KDEs is paramount.
Future-Proofing for All Inventory: Distributors should design their new traceability system with scalability in mind. Rather than implementing this rule for FTL items only, it is strategically sound to build a platform that can accommodate:
- Expanded FTL: The FDA is expected to expand the FTL over time.
- Non-Food Perishables: Implementing TLC and CTE logic for all expiration-sensitive stock, such as temperature-controlled pharmaceuticals or time-sensitive industrial chemicals, will preempt future regulation and significantly improve inventory management.
Conclusion
By embracing the spirit of FSMA 204—which prioritizes rapid, data-driven response to public health risks—distributors of all expiration-sensitive products can transform a compliance mandate into a powerful competitive advantage. LaceUp Solutions offers DSD, Route management, and WMS that can support Food traceability strategies. Contact us for more information on our solutions.
I hope this article have been helpful. I will continue to post information related to management, distribution practices and trends, and the economy in general. Our channel has a lot of relevant information. Check out this video on the subject.


Sorry, the comment form is closed at this time.