
What is an FDA inspection and how to be prepared for it?
One of the biggest threats to the disruption of a manufacturing or distribution company’s operation stems from an FDA inspection. In this article, I will explain what the FDA is, why it inspects companies, who is subject to inspection, and how to prepare to ace an inspection.
What is an FDA inspection?
The Food and Drug Administration (FDA) is required by law to conduct inspections and assessments of regulated facilities, such as food, drugs and cosmetics, to determine a company’s compliance with applicable laws and regulations. Inspection visits are scheduled in advance and might be triggered by a routinely scheduled investigation, survey, or response to a reported problem.
The FDA inspection consists of three steps:
- The introduction. The FDA investigator will present his credentials and the “Notice of Inspection” (FDA Form 482).
- Inspection and audit. The investigator will examine your production process, look at your quality management system (QMS) and collect samples. The audit part of the QMS includes many important topics that will delved into: quality policy, internal audit procedures, quality plan, product recall and rejection reports, production equipment maintenance, and training procedures.
- Closeout meeting. Upon completion of the inspection, the investigator will discuss any significant findings and concerns with your company’s management, and leave a written report of any conditions or practices, that, in the investigator’s judgment, indicate objectionable conditions or practices. This “Inspection Observations” list, also called FDA Form 483, can be used by your firm’s management as a guide to corrective action.
Who can be inspected and why?
Any company that sells food or drugs to retailers must comply with FDA regulations and is required to be inspected by the FDA. This applies to manufacturers, importers, brokers, distributors and wholesalers. Even producers of these types of goods in other countries must be approved by the FDA before being allowed to export to the USA.
How to prepare for an FDA inspection?
There are many guides that advice how to prepare for an FDA inspection, one of the best is the ¨FDA Inspection Guide”; but it all boils down to a single point: PLANNING. By knowing what you must comply with and the mechanics of the inspection visit, you can prepare to ace the FDA inspection. Here is a summary of the points you need to work with.
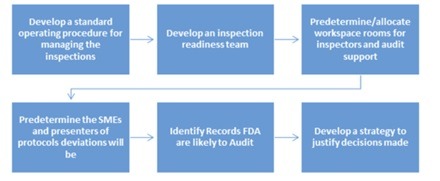
- Identify the documents that the FDA inspector will audit and prepare them in advance. Readiness to the inspector requests is crucial as it shows you know your responsibilities and are prepared. If you have any doubts there are many people that offer counseling on the matter.
- If you manufacture products make sure to have a sound maintenance plan and enforce its execution.
- Have a “Recall Plan” in place. A recall is an extreme situation where you are forced to retire products from lots that were found to be defective during the inspection. If that happens, the reaction speed you show is vital. The only way to quickly respond to a recall is with a WMS that allows product tracking by lot.
- If possible, conduct a mockup inspection with someone who plays the role of an FDA investigator and has the devil’s advocate mentality. This will allow you to detect weak points and develop strategies to defend yourself.
- Develop the procedure to manage the inspection. Who will be the main point of contact? Which team will be at the closeout meeting?
What to do if something goes wrong?
The closing meeting is the climax of the inspection. If you were well prepared, there probably will not be any serious observations from the inspector. But if you failed at any point, the FDA investigator will issue a 483 form detailing the issues you must address. The best case scenario occurs if you can correct the issues in the 483 during the meeting. That is why it is of the outmost importance that the key people of the organization be present at the closing meeting. The FDA gives 15 days to respond to observations that were not addressed at the meeting or that require action on your side.
There are three types of actions that the FDA inspector might indicate.
- No Action Indicated (NAI): The investigator did not find any observations.
- Voluntary Action Indicated (VAI): It means that, although the company deviates from some regulatory requirements, they are not that significant and the agency believes that the firm can handle them.
- Official Action Indicated (OAI): Some kind of official action is forthcoming, e.g., a warning letter, an injunction, or even a prosecution. This is the worst case scenario and could lead to temporary closure, denial of permission to import or produce a product.
I hope this article has been helpful to you. I will continue posting information related to warehouse management, distribution practices and trends, and the economy in general. If you are interested in this article or want to learn more about Laceup Solutions, please subscribe to stay updated on future articles.
There is a lot of relevant information on our channel. Check out this video about a customer’s experience with the FDA inspection.


Sorry, the comment form is closed at this time.